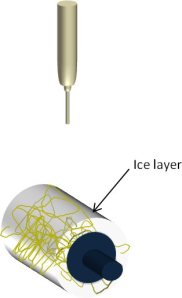
To avoid any contamination of the membrane when using a temporary scaffolding to give the membrane bulk, ice particles may be used. In a relatively humid environment, ice crystals will form on very cold surfaces. By using a collector that has been cooled down to a low temperature, fibers that deposit on that surface will also quickly be covered with a layer of ice. The layer of ice will act as temporary scaffolding to separate the nanofibers. Once sufficient nanofiber volume is constructed, the ice may be removed by simply allowing it to melt or through free-drying. This process has been shown quantitatively to increase the pore size of the resultant fibrous structure compared to conventional electrospinning. Simonet et al showed that the spatial fiber distance of polycaprolactone and polylactide fibers were increased from a range of 0 - 50 µm for conventional electrospun membrane to 50 - 200 µm using ice. Dry ice (frozen carbon dioxide) has been used successfully to cool the collector surface for this technique [Simonet et al 2007, Schneider et al 2009, Kim et al 2014].
In most cases, rapid cooling of the nanofiber surface encourages rapid formation of ice crystals on the surface of the nanofiber before the incoming nanofiber rests on it. Kim et al (2014) showed that using different solvents for electrospinning of the poly(lactic-co-glycolic acid) nanofiber with dry ice gives different level of compaction and pore sizes. Using 1,1,1,3,3,3,-hexafluoro-2-propanol (HFIP) as solvent results in similar membrane thickness with and without the use of dry ice. However, when tetrahydrofuran/dimethylformamide (THF/DMF) solvent mixture was used, the nanofibrous structure spun with the use of dry ice showed loose connections between fibers and large voids which was very different from the compact membrane spun without the use of dry ice. Although Kim et al (2014) said that HFIP have the lowest vapor pressure among the solvents compared, it was unclear whether that has an impact on the level of compaction in the nanofiber membrane.
Separate studies by Leong et al (2009) and Simonet et al (2014) using poly(lactic acid) and ice crystal formation on electrospun scaffold gave very different structures. While Simonet et al reported large thickness and inter fiber distance, Leong et al reported dimpled structures without significant increment in thickness. While acknowledging that experimental parameters and procedural differences may play a part in their different observation, a distinct difference is Leong et al used HFIP as solvent to dissolve poly(lactic acid) while Simonet et al used chloroform.
The level of compaction between the nanofibers using this process is dependent on two main factors. The first being the speed of water crystal formation and growth and the second is the speed at which the fibers are being laid. The speed of water crystal formation and growth is dependent on the temperature of the collector and the humidity of the electrospinning environment. Bulysheva et al (2012) using dry ice as the coolant on the collector recommended a relative humidity above 30% to create electrospun silk scaffolds with sufficient pore size to allow cell infiltration. At humidity below this level, the pore size appeared to be similar to conventional electrospun scaffold. It is unlikely that the type of solvent used in electrospinning will have an impact on the ice crystal formation and growth. However, the type of solvent may influence the electrospinning jet velocity due to the amount of charges it carries. For the results from Kim et al (2014), it is possible that HFIP encourages faster deposition of nanofiber on the collector and this reduces the amount and size of ice crystal formation over the nanofibers.
Stiffness of the polymer has been suggested as a possible factor in influencing the volume and pore size of the structure constructed using ice crystals. Simonet et al (2014) compared the pore size and porosity of electrospun polylactide and polycaprolactone using this process and showed that the stiffer polylactide gives a larger spatial fiber distance. More studies need to be carried out to investigate the parameters that affect the formation of 3D nanofibrous structure using this process. Nevertheless, it is likely that there is a limitation to the thickness of the membrane fabricated using this technique as the insulating property of nanofibers reduces the effectiveness of ice particle formation as the thickness increases.
In applications such as tissue scaffold, it is beneficial to have large inter-fiber pore size to support cell infiltration. Crouch et al (2023) successfully demonstrated the benefits of cryoelectrospinning to construct a trabecular scaffold. Using a mandrel packed with dry ice, ice crystals were formed on the surface as poly(ε-caprolactone) (PCL) fibers deposited on it. The formed structure was placed in a vacuum desiccator immediately to remove the water without structural collapse. Freeze drier may also be used for the same purpose. Standard electrospun PCL scaffold had a densely packed structure with small pore sizes while cryoelectrospun PCL scaffold had a more open structure with larger pore sizes. Transformed human normal trabecular meshwork 5 (NTM5) cell line cultured on both standard electrospun PCL scaffold and cryoelectrospun PCL scaffold showed cell penetration up to 37.39 µm in the latter while the former had cell penetration of 3.41 µm only. Cell attachment and infiltration of NTM5 cells were found to reach 62.31% of the total scaffold thickness hence demonstrating the advantage of using cryoelectrospinning for the construction of regenerative scaffolds.
Published date: 27 August 2012
Last updated: 01 April 2025
▼ Reference
-
Bulysheva A A, Bowlin G L, Klingelhutz A J, Yeudall W A. Low-temperature Electrospun Silk Scaffold for In Vitro Mucosal Modeling. J Biomed Mater Res A 2012; 100: 757.
Open Access
-
Crouch DJ, Sheridan CM, Behnsen JG, D'Sa RA, Bosworth LA. Cryo-Electrospinning Generates Highly Porous Fiber Scaffolds Which Improves Trabecular Meshwork Cell Infiltration. Journal of Functional Biomaterials. 2023; 14(10):490
Open Access
-
Kim H L, Lee J H, Seo H J, You K E, Lee M H, Park J C. Fabrication of Three-Dimensional Poly(lactic-co-glycolic acid) Mesh by Electrospinning Using Different Solvents with Dry Ice. Macromolecular Research 2014; 22: 377.
-
Leong M F, Rasheed M Z, Lim T C, Chian K S. In vitro cell infiltration and in vivo cell infiltration and vascularization in a fibrous, highly porous poly(D,L-lactide) scaffold fabricated by cryogenic electrospinning technique. J Biomed Mater Res 2009; 91A: 231.
-
Simonet M, Schneider O D, Neuenschwander P, Stark W J (2007) Ultraporous 3D polymer meshes by low-temperature electrospinning: Use of ice crystals as a removable void template. Polym. Eng. Sci. 47 pp. 2020
-
Simonet M, Stingelin N, Wismans J G F, Oomens C W J, Driessen-Mol A, Baaijens F P T. Tailoring the void space and mechanical properties in electrospun scaffolds towards physiological ranges. J. Mater. Chem. 2014; 2: 305.
-
Schneider O D, Weber F, Brunner T J, Loher S, Ehrbar M, Schmidlin P R and Stark W J (2009) In vivo and in vitro evaluation of flexible, cottonwool-like nanocomposites as bone substitute material for complex defects. Acta Biomater. 5 1775-1784
▲ Close list
 ElectrospinTech
ElectrospinTech