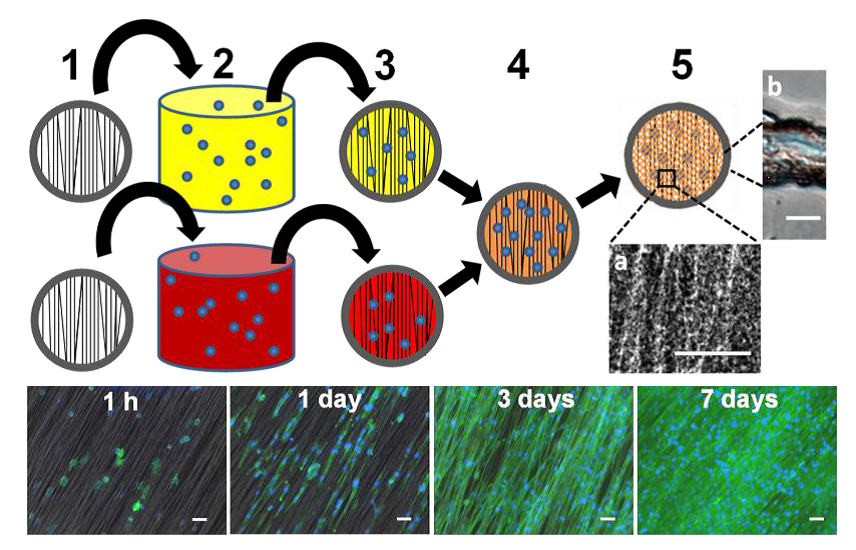
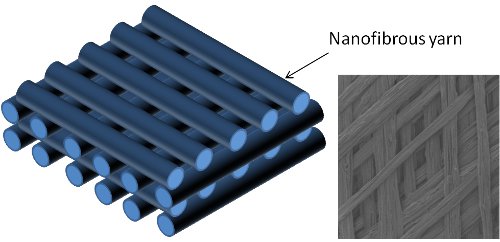
▼ Reference
- Beachley V, Hepfer R G, Katsaneviakis E, Zhang N, Wen X. Precisely Assembled Nanofiber Arrays as a Platform to Engineer Aligned Cell Sheets for Biofabrication. Bioengineering 2014; 1: 114. Open Access
- Cai Y Z, Zhang G R, Wang L L, Jiang Y Z, Ouyang H W, Zou H X. Novel biodegradable threedimensional macroporous scaffold using aligned electrospun nanofibrous yarns for bone tissue engineering. J Biomed Mater Res Part A 2012; 100A: 1187–1194.
- Deng M, Kumbar S G, Nair L S, Weikel A L, Allcock H R, Laurencin C T. Biomimetic Structures: Biological Implications of Dipeptide-Substituted Polyphosphazene-Polyester Blend Nanofiber Matrices for Load-Bearing Bone Regeneration. Adv Func Mater 2011; 21: 2641
- He X, Feng B, Huang C, Wang H, Ge Y, Hu R, Yin M, Xu Z, Wang W, Fu W, Zheng J. Electrospun gelatin/polycaprolactone nanofibrous membranes combined with a coculture of bone marrow stromal cells and chondrocytes for cartilage engineering. International Journal of Nanomedicine 2015; 10: 2089. Open Access
- Hong J K, Bang J Y, Xu G, Lee J H, Kim Y J, Lee H J, Kim H S, Kwon S M. Thickness-controllable electrospun fibers promote tubular structure formation by endothelial progenitor cells. International Journal of Nanomedicine 2015; 10: 1189. Open Access
- Inanc B, Arslan Y E, Seker S, Elcin A E and Elcin Y M (2009) Periodontal ligament cellular structures engineered with electrospun poly(DL-lactide-co-glycolide) nanofibrous membrane scaffolds. J. Biomed. Mater. Res. 90A 186-195.
- Joshi V, Lei N Y, Walthers C M, Wu B, Dunn J C Y. Macro-porosity enhances vascularization of Electrospun Scaffolds. J Surg Res 2013; 183: 18. Open Access
- Kosowska K, Domalik-Pyzik P, Krok-Borkowicz M, Chlopek J. Polylactide/Hydroxyapatite Nonwovens Incorporated into Chitosan/Graphene Materials Hydrogels to Form Novel Hierarchical Scaffolds. Int J Mol Sci. 2020 Apr; 21(7): 2330. Open Access
- Leung L H, Fan S, Naguib H E. Fabrication of 3D Electrospun Structures from Poly(lactide-co-glycolide acid)-Nano-Hydroxyapatite Composites. J Polym Sci Part B: Polym Phy 2012; 50: 242.
- Madurantakam P A, Rodriquez I A, Garg K, McCool J M, Moon P C, Bowlin G L. Compression of Multilayered Composite Electrospun Scaffolds: A Novel Strategy to Rapidly Enhance Mechanical Properties and Three Dimensionality of Bone Scaffolds. Advances in Materials Science and Engineering 2013; 561573, Open Access
- Orr S B, Chainani A, Hippensteel K J, Kishan A, Gilchrist C, Garrigues N W, Ruch D S, Guilak F, Little D. Aligned multilayered electrospun scaffolds for rotator cuff tendon tissue engineering. Acta Biomater. 2015 Article in press
- Pham Q P, Sharma U, Mikos A G. Electrospun Poly(E-caprolactone) Microfiber and Multilayer Nanofiber/Microfiber Scaffolds: Characterization of Scaffolds and Measurement of Cellular Infiltration. Biomacromolecules 2006; 7: 2796.
- Rashid M, Dudhia J, Dakin S G, Snelling S J B, Godoy R, Mouthuy P A, Smith R K W, Morrey M, Carr A J. Histopathological and immunohistochemical evaluation of cellular response to a woven and electrospun polydioxanone (PDO) and polycaprolactone (PCL) patch for tendon repair. Scientific Reports 2020; 10: 4754. Open Access
- Srouji S, Kizhner T, Suss-Tobi E, Livne E and Zussman E (2007) 3-D Nanofibrous electrospun multilayered construct is an alternative ECM mimicking scaffold. J. Mater. Sci. Mater. Med. 19 pp.1249
- Tzezana R, Zussman E and Levenberg S (2008) A Layered Ultra-Porous Scaffold for Tissue Engineering, Created via a Hydrospinning Method. Tissue Engin. 14 281-288.
- Vaquette C, Copper-White J. A simple method for fabricating 3-D multilayered composite scaffolds. Acta Biomaterialia 2013; 9: 4599.
- Wright L D, Young R T, Andric T, Freeman J W. Fabrication and mechanical characterization of 3D electrospun scaffolds for tissue engineering. Biomed Mater 2010; 5: 055006.
- Xu T, Binder K W, Albanna M Z, Dice D, Zhao W, Yoo J J, Atala A. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication 2013; 5: 015001.
- Yang X, Shah J D and Wang H (2009) Nanofiber Enabled Layer-by-Layer Approach Toward Three-Dimensional Tissue Formation. Tissue Engin. A 15 945-956.
- Yang M, Gao X, Shen Z, Shi X, Lin Z. Gelatin-assisted conglutination of aligned polycaprolactone nanofilms into a multilayered fibre-guiding scaffold for periodontal ligament regeneration. RSC Adv. 2019; 9: 507. Open Access
- Yang Y, Wimpenny I. Ahearne M (2011) Portable nanofiber meshes dictate cell orientation throughout three-dimensional hydrogels. Nanomedicine: Nanotechnology, Biology and Medicine 7, pp. 131-136
- Yun J W, Heo S Y, Lee M H, Lee H B. Evaluation of a poly(lactic-acid) scaffold filled with poly(lactide-co-glycolide)/hydroxyapatite nanofibres for reconstruction of a segmental bone defect in a canine model. Veterinarni Medicina 2019; 64: 531. Open Access
▼ Credit and Acknowledgement
Author
Wee-Eong TEO View profile
Email: weeeong@yahoo.com
 ElectrospinTech
ElectrospinTech