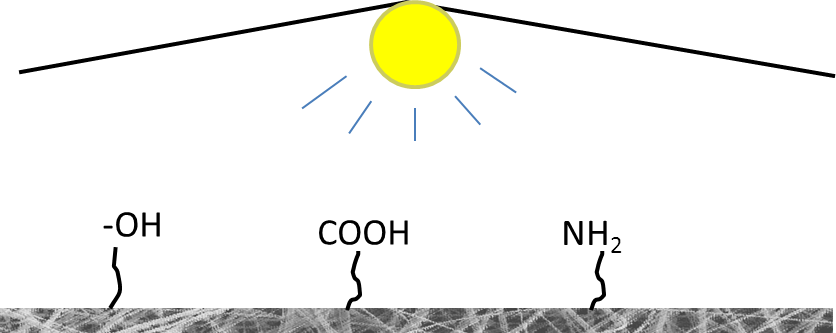
▼ Reference
- Cheng Q, Lee B L P, Komvopoulos K, Yan Z, Li S. Plasma Surface Chemical Treatment of Electrospun Poly(L-Lactide) Microfibrous Scaffolds for Enhanced Cell Adhesion, Growth, and In?ltration. Tissue Engineering: Part A 2013; 19: 1188.
- Esmail A, Pereira JR, Zoio P, Silvestre S, Menda UD, Sevrin C, Grandfils C, Fortunato E, Reis MAM, Henriques C, Oliva A, Freitas F. Oxygen Plasma Treated-Electrospun Polyhydroxyalkanoate Scaffolds for Hydrophilicity Improvement and Cell Adhesion. Polymers. 2021; 13(7):1056. Open Access
- Hoy C F O, Kushiro K, Yamaoka Y, Ryo A, Takai M. Rapid multiplex microfiber-based immunoassay for anti-MERS-CoV antibody detection. Sensing and Bio-Sensing Research 2019; 26: 100304. Open Access
- Kaur S, Ma Z, Gopal R, Singh G, Ramakrishna S, Matsuura T. Plasma-Induced Graft Copolymerization of Poly(methacrylic acid) on Electrospun Poly(vinylidene fluoride) Nanofiber Membrane. Langmuir 2007; 23: 13085.
- Koh H S. Polymeric Nanofiber Conduits for Peripheral Nerve Regeneration. PhD Thesis. National University of Singapore 2009 Open Access
- Ma Z, Ramakrishna S. Ce(IV)-Induced Graft Copolymerization of Methacrylic Acid on Electrospun Polysulphone Nonwoven Fiber Membrane. J Appl Polym Sci 2006; 101: 3835.
- Ma Z, He W, Yong T, Ramakrishna S. Grafting of Gelatin on Electrospun Poly(caprolactone) Nanofibers to Improve Endothelial Cell Spreading and Proliferation and to Control Cell Orientation. Tissue Engineering 2005; 11: 1149.
- Park K, Jung H J, Kim J J, Ahn K D, Han D K, Ju Y M. Acrylic Acid-Grafted Hydrophilic Electrospun Nanofibrous Poly(L-lactic acid) Scaffold. Macromolecular Research 2006; 14: 552.
- Robinette E J, Palmese G R. Synthesis of polymer-polymer nanocomposites using radiation grafting techniques. Nuclear Instruments and Methods in Physics Research B 2005; 236: 216.
- Wei Q F, Gao W D, Hou D Y, Wang X Q. Surface modification of polymer nanofibres by plasma treatment. Applied Surface Science 2005; 245: 16.
- Yan D, Jones J, Yuan X, Xu X, Sheng J, Lee J C M, Ma G, Yu Q. Plasma Treatment of Random and Aligned Electrospun PCL Nanofibers. Journal of Medical and Biological Engineering 2013; 33: 171. Open Access
▼ Credit and Acknowledgement
Author
Wee-Eong TEO View profile
Email: weeeong@yahoo.com
 ElectrospinTech
ElectrospinTech