▼ Reference
- Dai Y, Chen J, Li H, Li S, et al. (2012) Characterizing the Effects of VPA, VC and RCCS on Rabbit Keratocytes onto Decellularized Bovine Cornea. PLoS ONE 7(11): e50114. doi:10.1371/journal.pone.0050114 Open Access
- Neergaard-Petersen S, Ajjan R, Hvas A-M, Hess K, Larsen SB, et al. (2013) Fibrin Clot Structure and Platelet Aggregation in Patients with Aspirin Treatment Failure. PLoS ONE 8(8): e71150. doi:10.1371/journal.pone.0071150 Open Access
- Oliveira AC, Garzón I, Ionescu AM, Carriel V, et al. (2013) Evaluation of Small Intestine Grafts Decellularization Methods for Corneal Tissue Engineering. PLoS ONE 8(6): e66538. doi:10.1371/journal.pone.0066538 Open Access
- Teo W E, Liao S, Chan C K, Ramakrishna S(2008) Remodeling of Three-dimensional Hierarchically Organized Nanofibrous Assemblies. Current Nanoscience vol. 4 pg. 361-369.
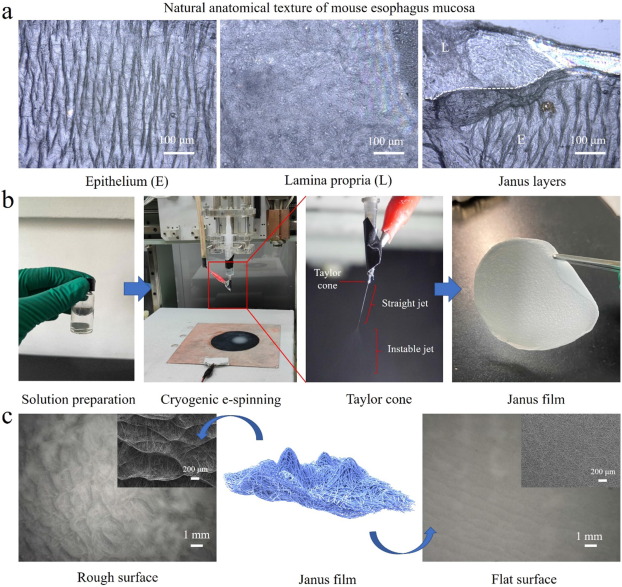
- Tian W, Liu X, Ren K, Fuh J Y H, Zhang X, Bai T, Wu B. Biomimetic Janus film fabricated via cryogenic electrospinning for gastrointestinal mucosa repair. Materials & Design 2023; 228: 111839. Open Access
- Ye X, Wang H, Zhou J, Li H, et al. (2013) The Effect of Heparin-VEGF Multilayer on the Biocompatibility of Decellularized Aortic Valve with Platelet and Endothelial Progenitor Cells. PLoS ONE 8(1): e54622. doi:10.1371/journal.pone.0054622. Open Access
- Youngstrom DW, Barrett JG, Jose RR, Kaplan DL (2013) Functional Characterization of Detergent-Decellularized Equine Tendon Extracellular Matrix for Tissue Engineering Applications. PLoS ONE 8(5): e64151. doi:10.1371/journal.pone.0064151 Open Access
- Zavagna L, Canelli E F, Azimi B, Troisi F, Scarpelli L, Macchi T, Gallone G, Labardi M, Giovannoni R, Milazzo M, Danti S. Electrospun Fiber-Based Tubular Structures as 3D Scaffolds to Generate In Vitro Models for Small Intestine. Macromolecular Materials and Engineering 2024; 10;: 2400123. https://onlinelibrary.wiley.com/doi/full/10.1002/mame.202400123 Open Access
▼ Credit and Acknowledgement
Author
Wee-Eong TEO View profile
Email: weeeong@yahoo.com
 ElectrospinTech
ElectrospinTech